Have you ever wondered why some reactions happen instantly, like the fizz of baking soda and vinegar, while others take a seemingly endless amount of time, like the rusting of a metal object? The difference lies in the speed at which these reactions occur, a characteristic known as the **rate of reaction**. And just like a perfect recipe needs the right ingredients and timing, chemical reactions are influenced by several critical factors that determine how quickly they proceed. Understanding these factors provides a deeper insight into the complex world of chemistry and unlocks the potential to control and optimize chemical processes.
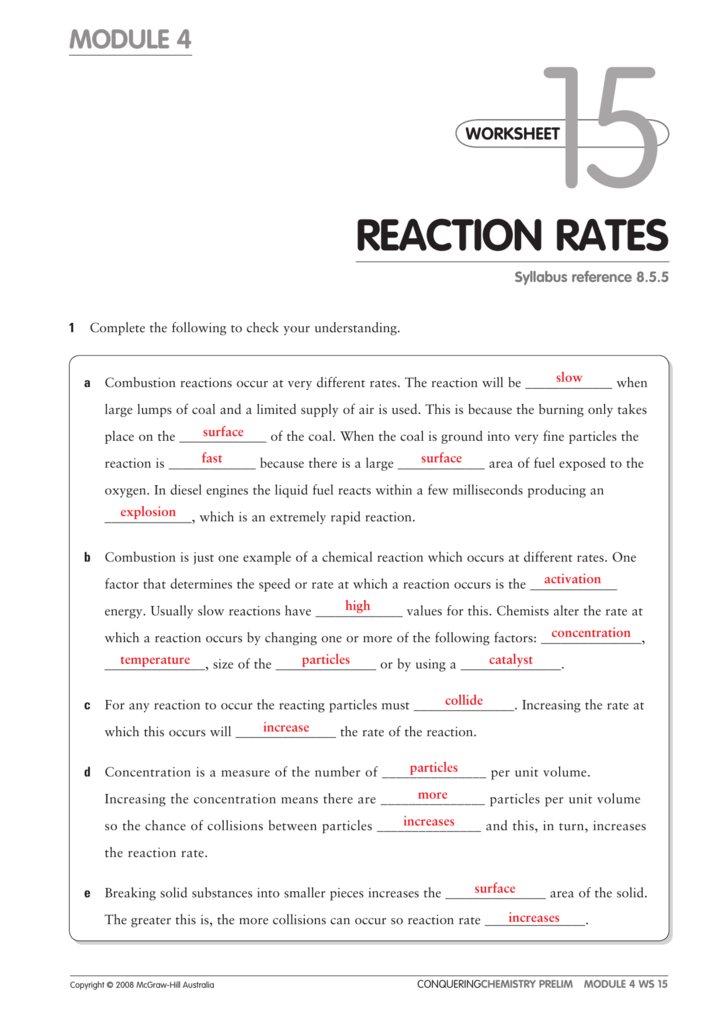
Image: printableschoolgabriele.z19.web.core.windows.net
Imagine you’re a chef trying to bake a cake. You know that adjusting the oven temperature or adding more baking soda can significantly change the outcome of your cake. Similarly, several factors can influence the rate of chemical reactions, determining how fast or slow they occur. In this article, we’ll delve into the major factors affecting the rate of chemical reactions and explore how they impact chemical processes. We will also discuss the practical implications of these factors and how they are utilized in various fields, from medicine to industrial production.
The Nature of Reactants
The Importance of Reactant Identity and Structure
The nature of the reactants is crucial in determining the rate of a chemical reaction. Different substances have inherent properties influencing their reactivity. For example, metals like sodium react vigorously with water, releasing a significant amount of heat, while gold remains inert. This difference stems from the electronic structures and bonding patterns of these elements. The arrangement of atoms within a molecule also plays a crucial role. More complex molecules with many bonds often react slower than simpler ones due to the need for multiple bonds to break and form.
The Role of Activation Energy
Every chemical reaction requires a certain amount of energy to initiate, known as the activation energy. This energy acts like a “barrier” that reactants must overcome to transform into products. Reactants with lower activation energies react faster as they need less energy input to reach the transition state. Activation energy is closely linked to the nature of reactants, as their electronic structure and bonding determine how easily they can overcome this barrier and form new bonds.

Image: www.coursehero.com
Concentration of Reactants
The Collision Theory
The rate of a chemical reaction is directly proportional to the concentration of reactants. This is explained by the collision theory, which states that reactions occur when molecules collide with sufficient energy and proper orientation. The higher the concentration of reactants, the more frequent these collisions are, leading to a faster reaction rate.
Illustrative Examples
Consider the reaction between hydrogen and oxygen to form water. Increasing the concentration of either hydrogen or oxygen will result in more collisions between the molecules, and hence a faster reaction rate. Similarly, in a combustion process, increasing the concentration of fuel or oxygen will lead to a more rapid combustion. This can be observed in the burning of a match, where the flame intensifies as more oxygen is supplied.
Temperature
The Energy-Rate Relationship
Temperature plays a significant role in determining the rate of a chemical reaction. As temperature increases, the kinetic energy of molecules also increases. This translates to more frequent and energetic collisions between molecules, ultimately increasing the likelihood of successful collisions that lead to product formation. Consequently, the rate of reaction usually increases exponentially with temperature.
A Practical Example
Imagine adding a drop of food coloring to a cold glass of water. You’ll notice that the color diffuses slowly. But as you warm the water, the color diffuses much faster. This is because the increased temperature leads to faster molecular motion and more frequent collisions between the food coloring molecules and the water molecules. Thus, the color spreads quickly throughout the water.
Surface Area
The Role of Interface
For heterogeneous reactions involving reactants in different phases (e.g., solid and liquid), the surface area of the solid reactant significantly influences the rate of reaction. The larger the surface area, the more contact points between the reacting substances, and the faster the reaction proceeds. This is because the reaction occurs only at the interface between the two phases.
Practical Implications
This concept is widely applied in various industrial processes. For instance, powdered iron reacts faster than solid iron with acids because the powdered form offers a much larger surface area. In combustion processes, finely divided fuels like sawdust burn faster and produce more heat than logs because they have a greater surface area exposed to oxygen. This principle is also crucial in catalysis, where catalysts with large surface areas are used to enhance reaction rates.
Catalyst
The Role of Catalysts
Catalysts are substances that accelerate the rate of a chemical reaction without being consumed in the process. They achieve this by providing an alternative reaction pathway with lower activation energy. By decreasing the activation energy, catalysts allow more molecules to overcome the energy barrier and react at a faster rate.
Ubiquitous Role in Chemical Processes
Catalysts play a vital role in many industrial chemical processes, including the manufacturing of fuels, pharmaceuticals, and plastics. For example, catalytic converters in automobiles use platinum and palladium catalysts to convert harmful pollutants in exhaust gases into less harmful substances. Enzymes, which are biological catalysts, are essential for all living organisms, enabling vital biochemical reactions to occur at the necessary speeds.
Tips and Expert Advice
Now that we’ve explored the main factors affecting the rate of chemical reactions, let’s discuss some practical tips for controlling and manipulating reaction rates. These tips can be particularly useful in laboratory settings and industrial applications.
To increase the rate of a reaction, you can:
- Increase the concentration of reactants. More reactants lead to more collisions and a faster reaction rate.
- Increase the temperature. Higher temperatures provide more energy for molecules to overcome the activation energy barrier.
- Increase the surface area. By breaking down reactants into smaller pieces, you increase the surface area exposed for reaction.
- Add a catalyst. Catalysts provide alternate reaction pathways with lower activation energies, speeding up the process.
On the other hand, to decrease the reaction rate, you can:
- Decrease the concentration of reactants. Fewer reactants mean fewer collisions and a slower reaction.
- Decrease the temperature. Lower temperatures reduce molecular motion and limit the number of successful collisions.
- Use a large piece of reactant (decrease surface area). This reduces the contact points between reactants, slowing down the reaction.
- Remove or deactivate the catalyst. Without the catalyst, the reaction will proceed at a slower rate.
FAQ
Q: How does light affect the rate of chemical reactions?
A: Light can affect the rate of some chemical reactions, particularly those involving photochemistry. Light can provide the energy necessary for the reaction to occur, or it can act as a catalyst. This is a common phenomena in reactions involving organic molecules and in photosynthesis, where light energy is used to convert carbon dioxide and water into sugars.
Q: Is there a specific formula to calculate the rate of a chemical reaction?
A: Yes, the rate law is used to express the relationship between the rate of a reaction and the concentrations of reactants. It is typically determined experimentally and takes the form: Rate = k[A]^m[B]^n, where k is the rate constant, and m and n are the orders of the reaction with respect to reactants A and B, respectively.
Q: What are some everyday examples of chemical reactions influenced by the factors discussed?
A: The rusting of iron is accelerated by the presence of oxygen and water, demonstrating the influence of concentration and the nature of reactants. Cooking a meal involves precise temperature control to optimize the reaction rates of various ingredients. Refrigeration slows down spoilage by reducing the rate of chemical reactions involved in food spoilage. These are just a few examples of how the factors discussed play a role in our everyday lives.
Factors Affecting The Rate Of Chemical Reactions Answer Key
Conclusion
Understanding the factors influencing the rate of chemical reactions is essential for various fields, from medicine and industry to everyday life. By controlling these factors, we can manipulate the speed of reactions to achieve desired outcomes. In this article, we’ve explored the key factors and explained their impact on reaction rates. We’ve also discussed practical tips for controlling these factors and provided examples of how they manifest in the real world.
Are you interested in learning more about the intricacies of chemical reactions and exploring other factors that influence their efficiency? Let us know your thoughts and questions below!






