The first time I encountered the concept of galvanic cells, I was completely baffled. It seemed like magic – how could a simple reaction between two metals produce electricity? The idea of using chemical energy to create electrical energy, a concept fundamental to today’s battery technology, was fascinating. This sparked my curiosity to delve deeper and understand the scientific principles behind this phenomenon. Through the classic “Experiment 32: Galvanic Cells” in a typical chemistry lab, I embarked on a journey to unravel the mystery and gain a practical understanding of how these cells work.

Image: www.thinkswap.com
This article aims to guide you through the process of conducting and analyzing “Experiment 32: Galvanic Cells.” This lab report is a key component of many introductory chemistry courses, and it serves as a stepping stone for understanding the foundations of electrochemistry. We’ll explore the key concepts, the experimental setup, how to interpret the results, and provide practical tips to maximize your understanding.
Understanding the Galvanic Cell: The Heart of Experiment 32
Defining the Galvanic Cell: A Bridge Between Chemistry and Electricity
At its core, a galvanic cell is an electrochemical device that converts chemical energy into electrical energy. It achieves this through a spontaneous redox reaction: a process involving the transfer of electrons between chemical species. The cell is designed to separate the oxidation and reduction half-reactions, allowing the electrons to flow through an external circuit, generating an electric current. A typical galvanic cell consists of two half-cells, each containing an electrode submerged in an electrolyte solution. The electrodes, usually made of different metals, act as sites for the electron transfer during the redox reaction.
The Dynamics of a Galvanic Cell: How Electrons Flow
The driving force behind the electron flow in a galvanic cell is the difference in standard reduction potentials between the two half-cells. The half-cell with the higher standard reduction potential has a greater tendency to gain electrons (reduction), while the other half-cell will lose electrons (oxidation). This difference in potential, known as the cell potential (E°cell), drives the movement of electrons from the anode (where oxidation occurs) to the cathode (where reduction occurs) through the external circuit.
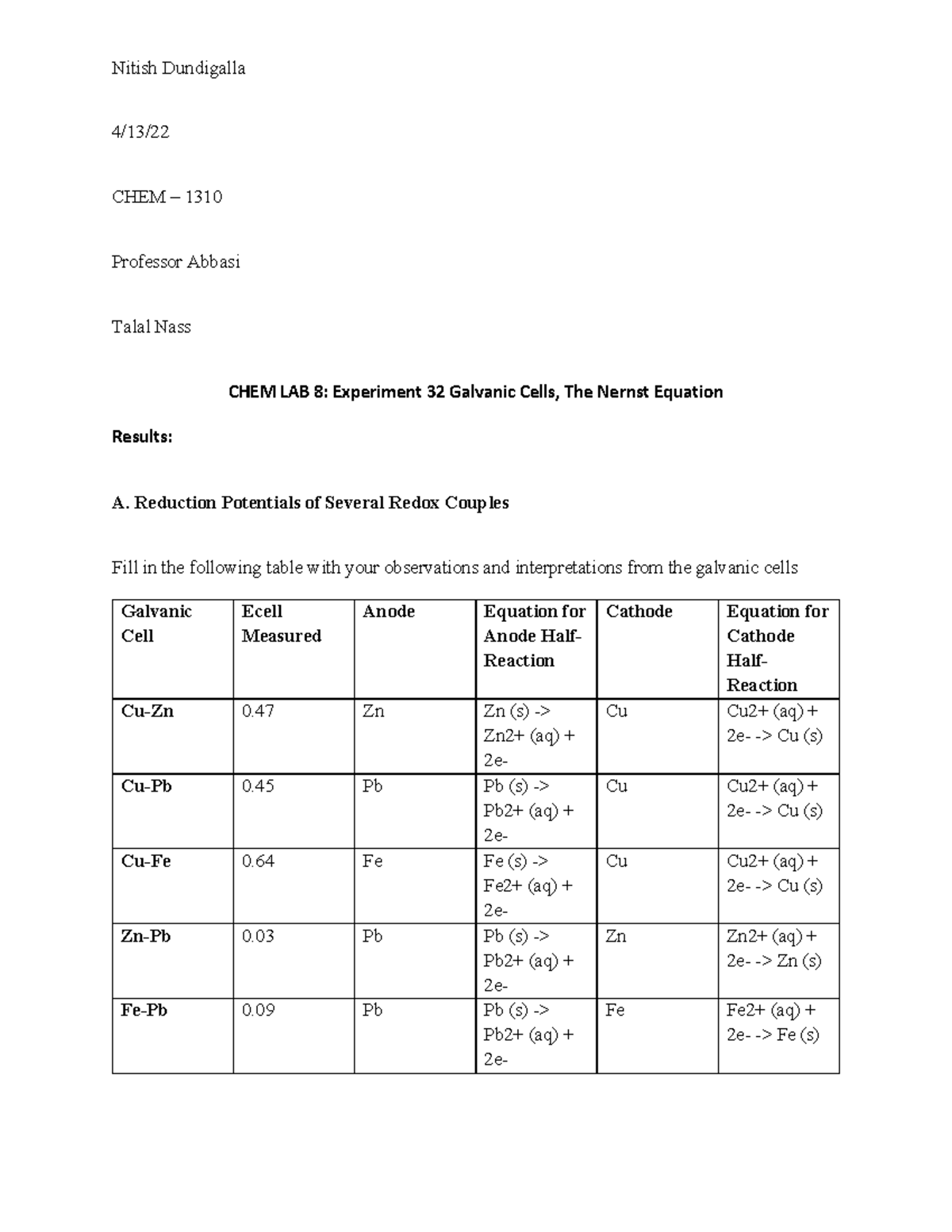
Image: www.studocu.com
Experiment 32: A Practical Introduction to Galvanic Cells
Experiment 32 is designed to illustrate the concepts of galvanic cells by building and testing a simple cell. Students typically use two different metal electrodes (like copper and zinc) and solutions of their respective ions (copper sulfate and zinc sulfate). The electrodes are connected through a wire, and the two solutions are linked via a salt bridge, which ensures electrical neutrality within the cell. By measuring the voltage across the electrodes, students can experimentally determine the cell potential and gain a deeper understanding of the electrochemical principles at play.
The experiment involves setting up the cell, carefully connecting the electrodes and the salt bridge, and using a voltmeter to measure the generated voltage. Students will observe that the voltage depends on the choice of metals and the concentration of the solutions. This hands-on experience helps solidify the theoretical concepts learned in class, providing valuable insights into the relationship between chemical reactions and electrical energy.
Interpreting the Results: Unveiling the Secrets of the Electrochemical Reaction
The analysis of Experiment 32 involves interpreting the measured voltage and identifying the half-reactions occurring at each electrode. Through understanding the standard reduction potentials and the direction of electron flow, students can deduce which metal acts as the anode (oxidation) and which acts as the cathode (reduction). By calculating the standard cell potential (E°cell) using the Nernst equation, students can further analyze the factors influencing the potential of the galvanic cell.
Emerging Trends in Electrochemical Research: Beyond the Basic Galvanic Cell
While Experiment 32 provides a fundamental introduction to galvanic cells, the field of electrochemistry has undergone substantial advances. Researchers are continuously exploring new materials, cell designs, and applications for these electrochemical systems. From fuel cells to batteries to electrocatalytic reactions, galvanic cell principles serve as a foundation for a wide range of emerging technologies.
Tips and Expert Advice for Mastering Experiment 32: Galvanic Cells
1. Ensure a Clean Setup: Preventing Contamination in Your Experiment
The accuracy of your results hinges on a clean and well-maintained experimental setup. Ensure all glassware and electrodes are thoroughly cleaned to avoid residues that can affect the cell’s performance. A clean environment minimizes the impact of external factors on your measurements.
2. Understand the Salt Bridge: A Crucial Connector in Galvanic Cells
The salt bridge plays a vital role in maintaining electrical neutrality within the cell. Choose a salt with relatively inert ions, such as potassium chloride, to avoid interfering with the redox reactions. A clean and functional salt bridge is essential for accurate voltage measurements.
3. Don’t Forget the Nernst Equation: Predicting Cell Potential
The Nernst equation is a powerful tool for predicting the cell potential under non-standard conditions, such as varying concentrations. Understanding the influence of concentration on cell potential will enhance your analysis of the experimental data and provide a deeper understanding of the system’s equilibrium.
Frequently Asked Questions (FAQs) About Experiment 32: Galvanic Cells
Q: What is the purpose of the salt bridge in a galvanic cell?
The salt bridge maintains electrical neutrality within the cell. Ions from the salt bridge migrate to the different half-cells to compensate for the charge buildup caused by the redox reactions.
Q. Why are the standard reduction potentials crucial in understanding galvanic cells?
The standard reduction potentials determine the direction of electron flow and the overall cell potential. They provide a framework for predicting which half-reaction will occur at the anode (oxidation) and which at the cathode (reduction).
Q: Could you explain the difference between a galvanic cell and an electrolytic cell?
A galvanic cell converts chemical energy into electrical energy through a spontaneous redox reaction, while an electrolytic cell uses electrical energy to drive a non-spontaneous redox reaction.
Q: What are some practical applications of galvanic cells?
Galvanic cells have many practical applications, including batteries, fuel cells, and corrosion prevention.
Experiment 32 Galvanic Cells Lab Report
Conclusion: From Experiment to Understanding
Experiment 32: Galvanic Cells is not just a lab report; it is an introduction to a world of fascinating electrochemical phenomena. By understanding the principles of galvanic cells, you gain insights into the fundamental connection between chemistry and electricity, paving the way for exploring more complex electrochemical systems and their applications in various fields. Are you interested in learning more about galvanic cells and their real-world applications? Share your thoughts in the comments below!






